Meet Iris Ding, Data Manager II
What really drives me in my role is knowing I'm making a meaningful impact.

Meet Iris. In the world of clinical research, where data meets patient care, this dedicated Iris, a Data Manager II has found her true calling. Driven by the meaningful impact of contributing to treatments that improve and save lives, she thrives where science, medicine, and technology meet. Her work ensures the quality and integrity of data that ultimately leads to new therapies for patients in need.
Beyond the satisfaction of patient impact, Iris values the professional growth opportunities in this dynamic field. The balance of structured processes within a regulated industry combined with the variety of different studies creates an engaging environment that blends stability with innovation—a perfect match for her. Find out more below.
Why did you choose the Clinical Research industry?
I chose the clinical research industry primarily because of the patient impact. I love contributing to treatments that improve and save lives, being part of the process that brings new therapies to patients in need, and making a difference in healthcare outcomes through quality data.
The scientific engagement aspect is also really appealing to me—working at the intersection of science, medicine, and technology, being involved in cutting-edge medical advancements, and continuously learning about different therapeutic areas and treatments keeps things interesting.
There's a meaningful purpose to this work that I find fulfilling. My daily tasks have clear societal value, and I'm part of solutions to significant medical challenges, which gives me a sense of purpose.
The professional growth opportunities are excellent too. There are diverse career paths within a growing global industry, the skills are transferable across multiple therapeutic areas, and I've been able to develop both technical and soft skills.
I also appreciate the balance of structure and dynamism in this field. We work in a regulated industry with clear processes, but still experience variety through different studies and therapeutic areas. It's a nice mix of stability and innovation
What does a day in the life of Data Manager II look like?
My typical day starts with checking emails to address any urgent data queries that have arisen overnight. I usually have few team meetings to discuss how our studies are progressing, review timelines, and talk through any challenges we're facing.
A big part of my day involves reviewing data metrics and dashboards to keep an eye on study progress and data quality. I frequently coordinate with our CRAs about site-specific data issues and update our data management plans or other study documentation as needed.
I also collaborate with our programmers on database specifications and edit checks, and conduct data review meetings with study teams to address ongoing issues. Sometimes I'll train site staff or colleagues on our data management processes and systems.
If we have upcoming database locks or interim analyses, I'll spend time preparing for those. And depending on where we are in a study lifecycle, I might be working on database set-up or revisions for new studies or system updates.
Throughout the day, I'm constantly communicating with sites to resolve outstanding queries, liaising with other teams like Biostatistics, Medical, and Clinical Operations, documenting processes, and making sure we're compliant with SOPs, regulatory requirements, and data standards.
It's busy, but I love knowing that my role is critical in ensuring data integrity and quality throughout the clinical trial process. At the end of the day, we're contributing to the development of new treatments for patients, which makes it all worthwhile.
As a Data Manager II what are you passionate about your role?
What really drives me in my role is knowing I'm making a meaningful impact. I contribute to patient care by ensuring the quality and integrity of data that ultimately leads to new treatments. Seeing studies through from start to finish and knowing my work directly impacts regulatory submissions and medical advancements gives me a real sense of purpose.
I also love the problem-solving aspects of my job. Resolving complex data issues that require critical thinking and investigative skills keeps me engaged, and I enjoy finding innovative solutions to streamline processes and improve data quality.
The technology side is exciting too. We work with cutting-edge Electronic Data Capture (EDC) systems and data visualization tools, and I'm constantly implementing new technologies that transform traditional data management approaches. Being at the forefront of digital transformation in clinical trials keeps things fresh and interesting.
I really value the cross-functional collaboration in my role. I've built relationships with diverse teams across the organization and learn so much from experts in various therapeutic areas and functional specialties.
There's also great opportunity for professional growth here. I'm constantly learning in this ever-evolving field with new regulations and technologies, and I've been able to develop expertise in specific therapeutic areas and data management specializations.
What skills are needed to be successful within Data Management?
To be successful in our team, you need a good mix of technical skills, process knowledge, soft skills, regulatory understanding, and leadership abilities.
- On the technical side, you definitely need to be proficient with EDC systems like Medidata Rave and have a solid understanding of database structures, queries, and data relationships. Some basic statistical knowledge helps when collaborating with biostatisticians, and at least a familiarity with programming concepts makes working with our programmers much easier. Advanced Excel skills are also essential for data manipulation and reporting.
-
Process-wise, you need expertise in data validation, efficient query management, and strong documentation skills. Being able to take a risk-based approach to identify critical data points and focus quality control efforts appropriately is crucial, as is the ability to translate clinical protocols into data collection requirements.
-
The soft skills are just as important though. Meticulous attention to detail, strong problem-solving abilities, and clear communication skills—especially explaining technical concepts to non-technical stakeholders—are all vital. You also need excellent time management to balance multiple studies and competing priorities, adaptability to quickly adjust to new systems or processes, and good teamwork skills for cross-functional collaboration.
-
Regulatory knowledge is non-negotiable in our field. Understanding GCP requirements, familiarity with FDA and other regulatory guidelines, knowledge of data privacy regulations, and maintaining audit-ready documentation are all essential.
-
As you progress, leadership skills become more important too—project management, mentoring junior team members, identifying process improvements, and strategic thinking about how data management fits into the broader clinical development process.
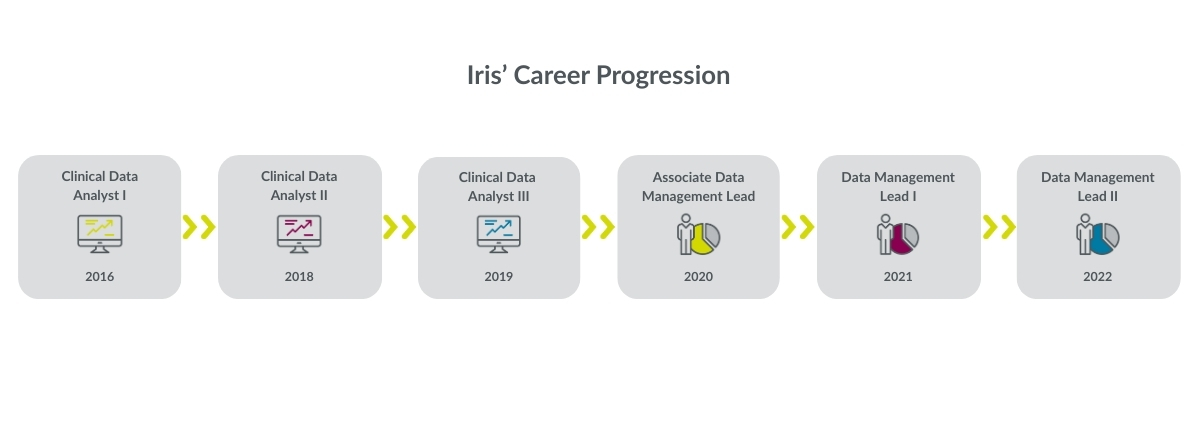
How has Parexel supported your career growth within Data Management?
Parexel has supported my growth in data management through clear progression paths from Associate Data Manager to Senior and Principal roles. I've had opportunities to specialize in specific therapeutic areas and data systems, which has deepened my expertise.
The company provides both technical and soft skills training relevant to advancing in data management, and I've gained exposure to increasingly complex studies and leadership responsibilities over time.
I've found that speaking openly with my manager about my career aspirations has been really helpful, as has connecting with longer-tenured colleagues about their growth experiences. Parexel has good internal career development platforms, and I've benefited from the mentorship programs available here.
I've participated in the Passport of Discovery program, which was a great learning experience that helped me develop new skills and broaden my understanding of the organization. Parexel's Passport to Discovery program is a career development initiative designed to provide hands-on experience in the clinical research industry. The program offers structured training, mentorship, and rotational assignments across different functional areas to build skills and knowledge in clinical research operations.
What Makes Parexel Standout
What have you experienced at Parexel but not anywhere else?
One thing that's really stood out to me at Parexel is the decentralized work mode. The company has a genuine commitment to work-life balance and remote work that I haven't experienced elsewhere.
Being part of one of the world's leading CROs gives me exposure to such diverse therapeutic areas and cutting-edge clinical trials that I don't think would be available at smaller organizations.
Parexel's emphasis on patient-centricity in clinical trials, including their Patient Innovation Center, is also quite unique.
I appreciate the specialized expertise in supporting biotech companies through Parexel Biotech, and the deep specialization in certain therapeutic areas has allowed me to develop focused expertise that I couldn't get elsewhere.
The academic partnerships through the Parexel Academy are something I haven't seen at other companies, and the truly global nature of the organization—with operations in over 50 countries—provides international exposure that's been invaluable for my professional development.
How do you work "With HeartTM" and what does it mean to you?
For me, working "With HeartTM " in my data management role means remembering that behind every data point is a real patient. I meticulously review data knowing that accuracy directly impacts patient safety. When resolving queries, I take time to understand site challenges rather than just pushing for quick resolutions.
I try to support my team members during high-pressure periods like database locks, because we're all in this together. When designing data collection methods, I consider the patient experience and how we can make participation less burdensome while still collecting quality data.
I make a point of celebrating team successes and acknowledging individual contributions, and I maintain work quality even when facing tight timelines because I know what's at stake.
How do you value Parexel keeping the patient at the heart of everything we do?
I think about patients in everything I do as a Data Manager. When designing data collection methods, I try to minimize patient burden while still gathering the necessary information. I ensure timely query resolution to maintain study integrity because delays can impact patients waiting for these treatments.
I implement rigorous data validation to protect patient safety, and I work to facilitate accurate analysis that leads to valid scientific conclusions. This patient-focused approach makes my work at Parexel more meaningful compared to organizations where financial metrics alone drive decisions.
It helps me remember that behind every data point is a real person, and the quality of my work directly impacts their care and potentially their lives. That's a responsibility I take very seriously, and it's one of the things I value most about Parexel's culture.
Getting Personal


What do you enjoy outside of work?
Outside of work, I really enjoy singing—it's a great creative outlet for me. And I love playing outside with my son. Those simple moments of connection and joy are what I look forward to after a busy day of managing data!
Explore Parexel
-
 Learn More
Learn MoreOur work culture
Learn about our culture, perks, learning opportunities, and our corporate responsibility approach. Parexel's compassionate and results-driven work culture prioritizes patient care and employee growth.
-
 Learn More
Learn MoreData Manager Qualifications, Skills and Experience
Discover the qualifications, education, experience, and skills needed to be a successful Data Manager.
-
 Learn More
Learn MoreMeet Patrycja, Data Manager II
Patrycja reflects on her career, motivations and how her actions demonstrate working with heart and always putting patients first.
Featured Jobs
- Senior Clinical Data Analyst (Home-based) - Poland, South Africa, Lithuania or UK - FSP Poland - Remote; Lithuania, Remote; South Africa, Remote; United Kingdom, Remote, Remote
- R0000038368 Manager, Centralized Data & Sample Management - Poland / Spain / Hungary - FSP Spain - Remote; Hungary, Remote; Poland, Remote, Remote
- Data Management Lead - San Francisco, California
Sign up for our Talent Community
Sign up and we’ll reach out with job alerts when positions that match your career interests become available. We’ll also share periodic updates about the latest company news and events.

